Meditalia Srl – We deliver Care
Over 40 Years of Expertise Experience at Your Service
The bio-medical district of Valtellina was born in the 70’s as the protagonist of the development of the Italian infusional sector. In 1980, Pierrel Hospital founded the current Lovero (So) production site specializing in the manufacture of compound medical grade materials for tubular, tubings and bags for hospital use.
Meditalia is an Italian company that has been operating for over 40 years in the production and sales of raw materials and finished products for use in the medical field. Our production site is located in Valtellina, north – Lombardia – in the province of Sondrio.









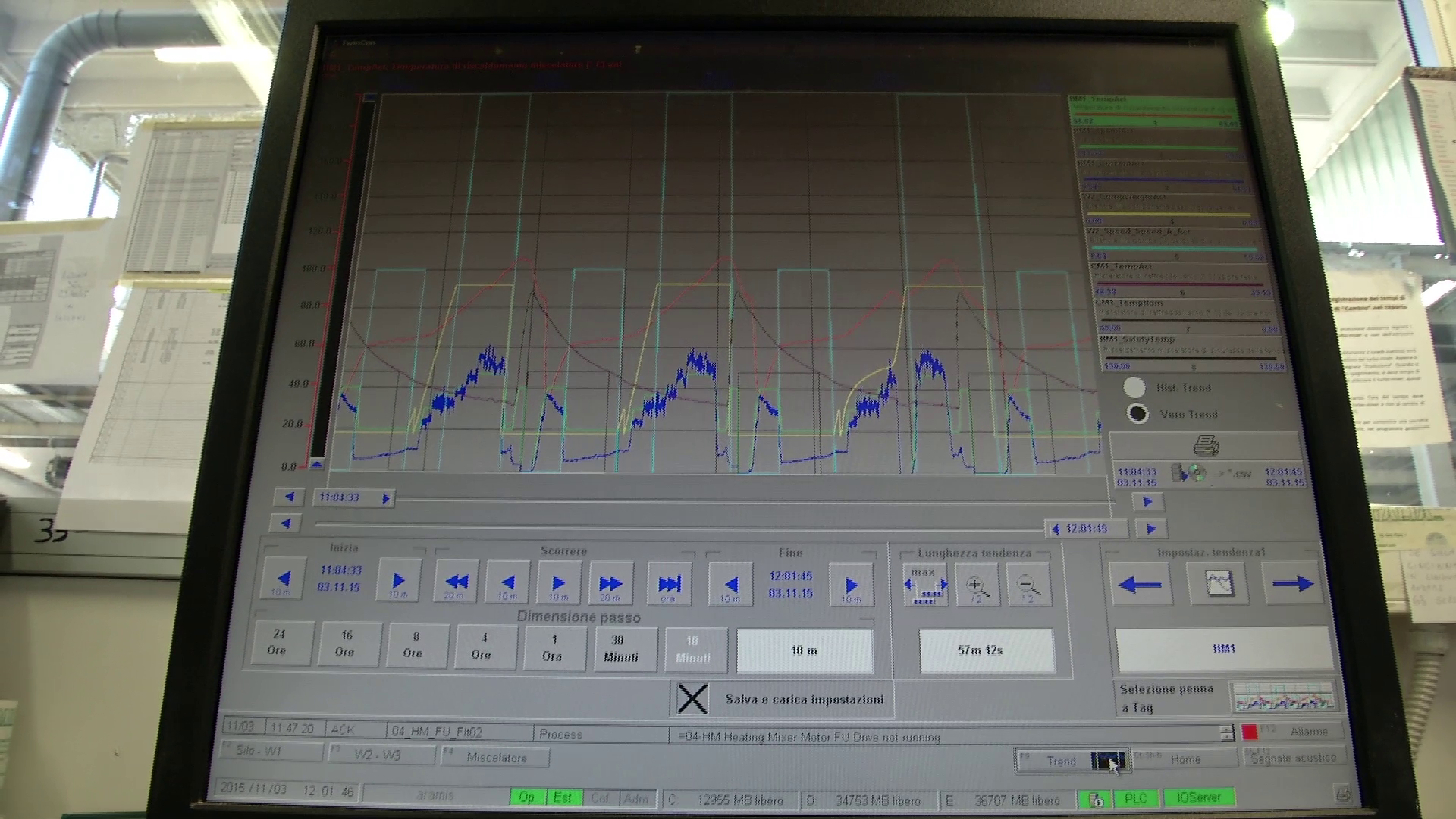









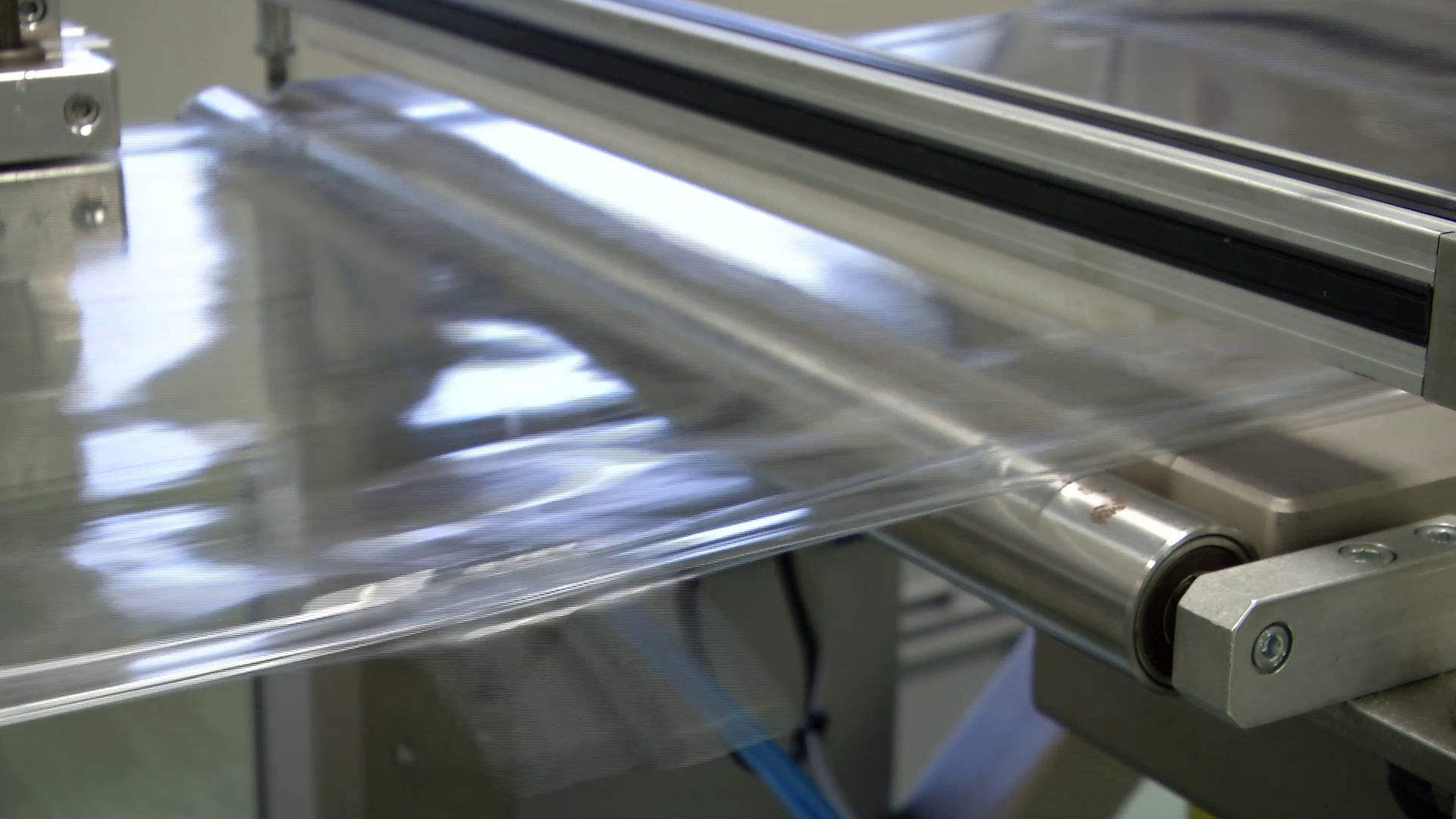








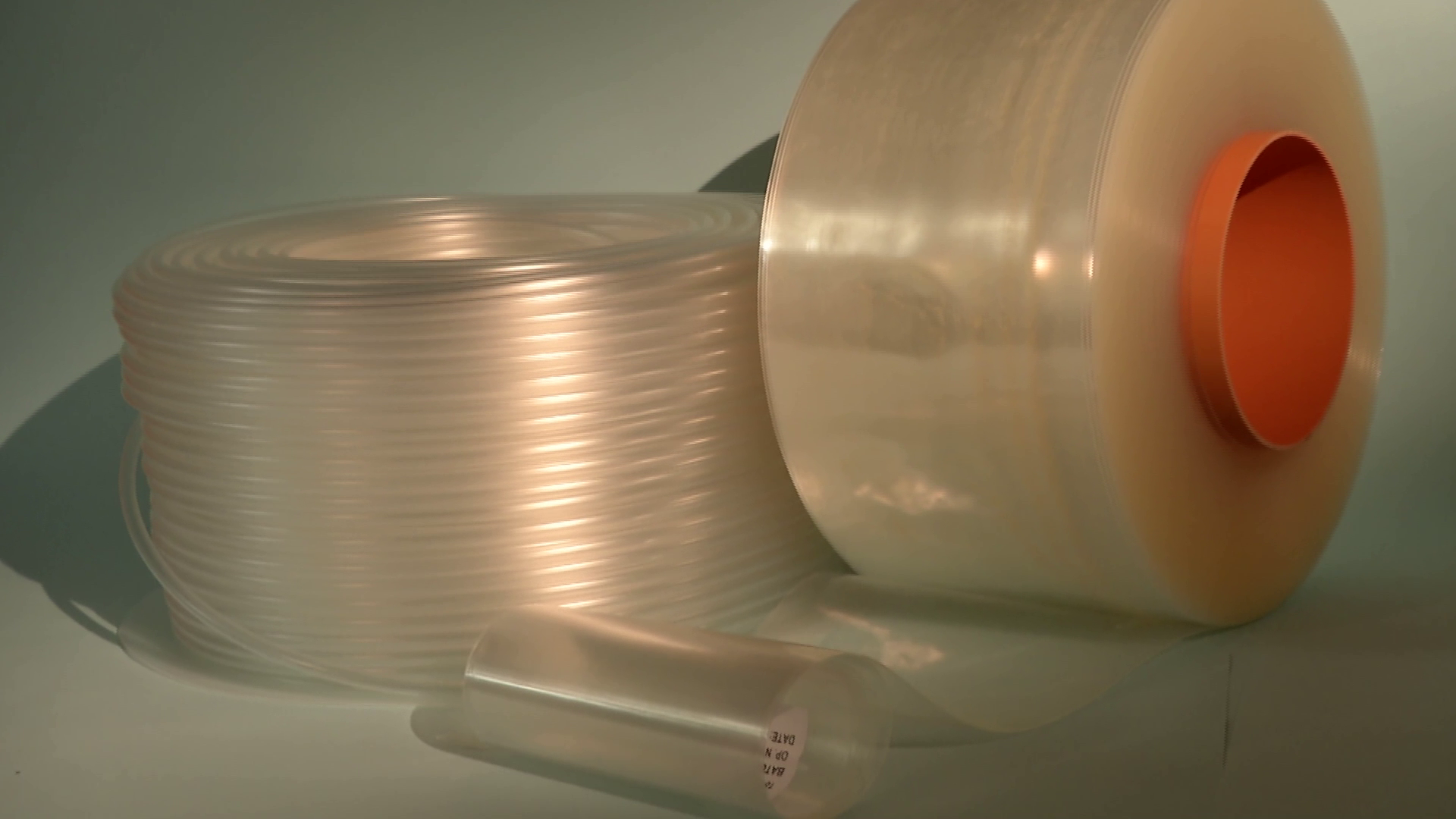
Starting from the production of PVC, PP, EVA grade plastic tubes and tubings – trademarks Solutran®, Solucare®, NutriEVA® – compliant with the requirements of International Pharmacopoeia and used by pharmaceutical companies and / or by processing companies working in the biomedical sector.
Meditalia is also present in the hospital market with its own branded CE products, such as:
- Blood Bags – Solutran®hemo
- Ozone self-therapy bags – Solutran®hemo O2O3 brand
- Bags for collecting and evaluating blood loss during childbirth – Kifarm®
- Multiple bags for the preparation and administration of the cordonal blood gel BioNest®
The experience and know-how achieved in nearly 40 years of activity allows us to offer our customers customized products to the market in accordance with the technical and dimensional specifications required.



 1984
1984  2017
2017 
